Application of cobalt-free materials in R-PSOCs
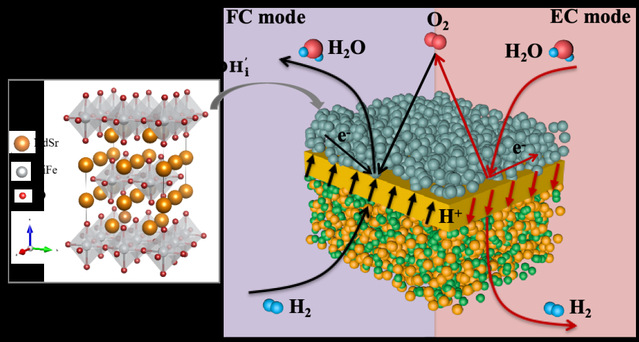
Reversible proton-conducting solid oxide cells (R-PSOCs) are electrochemical devices capable of directly converting the chemical energy of hydrogen into electrical energy and vice versa. These cells operate at lower temperatures, typically within the range of 500-700°C, which makes them more attractive for commercialization. A key challenge hindering the commercialization of R-PSOCs lies in the catalytic activity and longevity of the air electrode. The air electrode serves as the site for oxygen reduction (ORR) and oxygen evolution reactions (OER), critical processes in the operation of R-PSOCs. At lower temperatures, the rate of these reactions decreases significantly, which impacts the overall performance and efficiency of the cell. To address low activity, the researcher proposed the use of triple ionic-electronic conductors (TIECs) in the air electrode. These materials are capable of conducting protons, oxygen ions, and electrons (holes under oxidizing conditions), offering a significant advantage. In TIECs, protons from the electrolyte can diffuse to the surface of the air electrode and engage directly in the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER), thereby activating the entire surface of the air electrode. While Co-containing materials have been reported to demonstrate high catalytic activity, they present a major drawback: their thermal expansion coefficients are significantly higher than those of the electrolyte. This mismatch in thermal mechanical properties exacerbates the risk of delamination between the air electrode and the electrolyte during thermal cycling, leading to performance degradation.
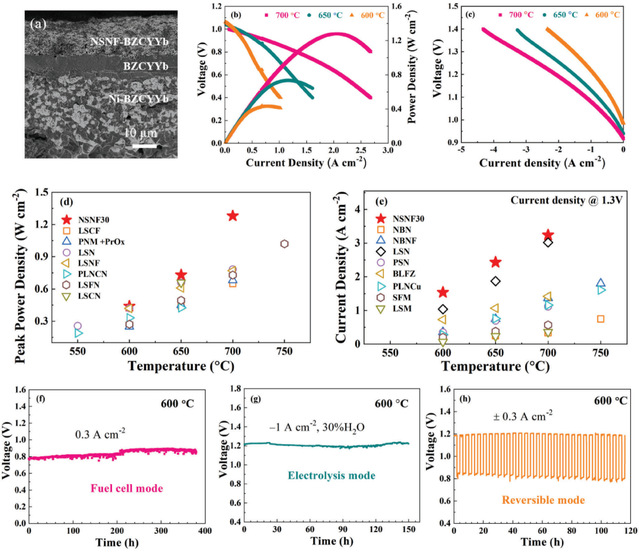
The fuel cell search team led by Professor Wang Shaorong at School of Chemical Engineering and Technology designed and developed a cobalt-free Ruddlesden-Popper structured material featuring Nd0.8Sr1.2Ni1-xFexO4±δ (NSNF), where x is varied across several values (0, 0.15, 0.3, 0.45, 0.55, 0.7, and 0.85). This material serves as a triple-phase conductor for the air electrode in R-PSOCs. By comprehensively analyzing the phase structure, electrical conductivity, thermal expansion coefficient, chemical adsorption, hydration properties, and electrocatalytic activity of the different Fe-doped compositions within this series, the team has identified an optimal composition, Nd0.8Sr1.2Ni0.7Fe0.3O4±δ (NSNF30). The exceptional electrochemical performance and stability of the NSNF30 air electrode when applied in R-PSOCs have been highlighted in a research paper titled “An Active and Stable Cobalt-free Ruddlesden-Popper Nd0.8Sr1.2Ni1-xFexO4±δ Air Electrode for Reversible Proton-conducting Solid Oxide Cells”. This study has been published in the prestigious international journal Advanced Functional Materials with an impact factor of 19. Chen Ting from School of Chemical Engineering and Technology is the first author. The corresponding authors include Professor Wang Shaorong from School of Chemical Engineering and Technology, Researcher Zhou Yucun from Beijing Huairou Lab, and Associate Professor Zhou Juan from Nanjing University of Science and Technology. China University of Mining and Technology is credited as the primary affiliation for this research.
In addition to their previous work, Professor Wang Shaorong and Dr. Chen Ting, in collaboration with Beijing Huairou Lab, have recently designed and developed a strontium-free and cobalt-free perovskite-based triple ionic-electronic conductor (e-/O2-/H+) air electrode material, Ba1-xCaxFeO3-δ (BCF), where x is varied among 0.1, 0.2, and 0.3. Their research revealed that introducing calcium (Ca) into the B-site of the perovskite lattice addresses the structural instability issues observed in BaFeO3 materials. The doping of Ca not only stabilizes the phase structure but also enhances the material's oxygen adsorption capacity and hydration catalytic ability, resulting in superior ORR or OER activity. Furthermore, the reversible protonic ceramic battery featuring BCF82 as the air electrode demonstrated exceptional electrochemical performance and stability. The results confirmed that the Ca-doped, strontium- and cobalt-free BCF material possesses outstanding ORR or OER activity alongside commendable durability, significantly advancing thedevelopment of R-PSOCs. This groundbreaking research, titled “A promising strontium and cobalt-free Ba1-xCaxFeO3-δ air electrode for reversible protonic ceramic cells”, has been published in the esteemed international journal Applied Catalysis B: Environmental and Energy, which holds an impact factor of 22.1. The article credits Dr. Zhang Guangjun from School of Chemical Engineering and Technology as the first author. Professor Wang Shaorong, Dr. Chen Ting from School of Chemical Engineering and Technology, and Researcher Zhou Yucun from Beijing Huairou Lab are acknowledged as co-corresponding authors. China University of Mining and Technology is recognized as the primary affiliation.
The aforementioned research has been generously supported by multiple national funding projects. The fuel cell research team has been dedicated to the study of electrode materials, single cells, and system designs for both efficient power generation and hydrogen production via water electrolysis. Their work is pivotal in supporting the realization of dual carbon goals—achieving peak carbon emissions and carbon neutrality.